ATP and ATP hydrolysis
To understand how motor proteins 'walk' around, one has to know what ATP and ATP hydorlysis is. ATP is a battery-like molecule, standard to every living organism. the phosphoanhydirde bonds between every phosphorus and an oxygen atom to its right has a high potential energy, or 'stored energy'. This energy is used to power countless actions in an organism.
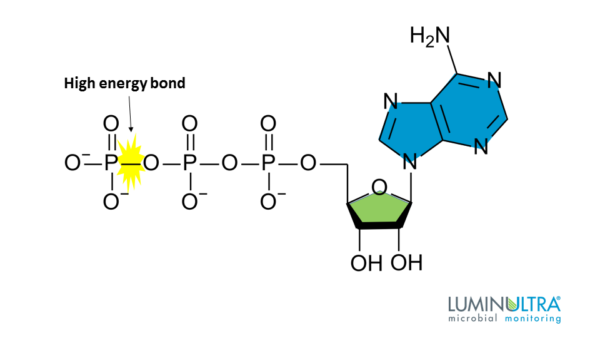
Hydrolysis is how the stored energy is released. When a water molecule interacts with ATP, a hydogen ion, an inorganic phosphate(Pi), and ADP(a molecule created when one Pi is separated from ATP) is made. This process disconnects a phosphoanhydirde bond, releasing the energy it contained.

Motor proteins
Motor proteins are essential in every cells' function. They move along the cytoplasm of the cell, transporting cargo or playing a role in muscle contraction.
Myosin
Myosins are motor proteins that carry out the process of muscle contraction. Muscle tissue is made up of thin muscle fiber, which is in turn made out of thin myofibril. Myofibril consists of sarcomeres, and in sarcomeres myosins and actins function for muscle contraction.


Sarcomeres consist of mainly two parts: myosin and actin. Actin is also called 'thin filament' while myosin is called 'thick filament', both due to their thickness. The Z line is where actin are attached to(in a Z-form), and the M line is basically the middle line vertical to the middle of a thick filament.
Muscle contraction process(myosin)
(1) The relaxed state of a myosin is when ATP is attacted to its head. At this 'resting state', the myosin head is closer to the thick filament.
(2) When a water molecule reacts with ATP, hydorysis occurs. The chemical process of ATP --> ADP + Pi happens, thus releasing energy. This energy is used to move the myosin head towards the thin filament, making it connect to a tropomyosin. This action also makes the actin(and the Z line) move towards the M line, resulting in the contraction of muscle.
(3) After process 2, "ADP + Pi" is released from the myosin head, and in its place a new ATP molecule is connected. The myosin head returns to its relaxed state, waiting for either hydrolysis and muscle contraction to happen again or the muscle to relax.
Kinesin
Kinesin are motor proteins that transport cargo throughout the cell. They move towards the (+) end of a microtuble, which means the outward direction from the nucleus. One of the differences between kinesin and other motor proteins(myosin and dynein) is that in a kinesin walking process, ATP hydorlysis is used to disconnect a motor domain with the microtuble.This is the opposite with dynein and myosin.
Kinesin walking process

(1) Both catalytic cores(yellow) are bound with ATP in their regular state. Until a motor domain connects to a microtuble, te kinesin drifts in the cell due to brownian motion. (stage 1&2 of illustration)
(2) ADP is released from the catalytic core connected to the microtuble. In its place, an ATP molecule is attached. Due to the new connection, the neck linker(orange line)'s position changes, wraping itself around the catalytic core, thus bringing the other motor domain forward. The other motor domain is then attached to another section of the microtuble. (stage 3 of illustration)
(3) ATP hydrolysis happens, freeing the earlier-connected motor domain(red outline). Pi is released, and the cycle starts again from stage 2 in illustration. (stage 4&2 of illustration)
Dynein
Dynein are motor proteins that also transport cargo throughout the cell. Opposite to kinesin, they move towards the (-) end of a microtuble, which means the direction towards the nucleus. Dynein has a structure called an ATPase ring. It is a row of several molecules folded together. In the illustration bellow, the ATPase is the big, round purple section.
Dynein walking process

(1) In a strong binding state, both ATPase rings are bound with ADP. When one of the ATPases disconnect with ADP and bind with ATP, its movement begins. (stage 5&1&2 of illustration)
(2) The binding of ATP lifts the stalk(long section) from the microtuble. It also changes the formation of the linker(pink) so that the stalk stretches out. The stalk then connects to another section of the microtuble. The movement of a dynein is very irregular and unpredictable: the step distance varies every time, and backward steps are frequent. (stage 2&3&4 of illustration)
(3) ATp hydrolysis causes a power stroke, both pulling the cargo towards the moving direction and strongly binding the stalk with the microtuble. Pi is released, but ADP is connected with the ATPase ring. The cycle repeats. (stage 4&5 of illustration)
Kinesin vs. Dynein
| Kinesin | Dynein | |
| Step Size | 8 nm | 8~32 nm |
| Step Type | Hand-over-hand |
Hand-over-hand, Inchworm |
| Backward Steps | Rare | Frequent |
| Direction | (+) end of microtuble(cell outward) | (-) end of microtuble(cell inward) |
| Use of ATP Hydroysis | Lifting motor domain from microtuble |
Binding motor domain to microtuble, Cargo tug towards moving direction |
'Science Notes > Biology' 카테고리의 다른 글
| On small movements of the eye(미세한 눈의 움직임) (0) | 2021.02.13 |
|---|
